Taylor JL et al. Uncoupling of Ca2+ sparks from BK channels in cerebral arteries underlies hypoperfusion in hypertension-induced vascular dementia.
Proceedings of the National Academy of Sciences USA. 2023;120(33):e2307513120. doi: 10.1073/pnas.2307513120.
Weblink to Open access text
Listen to Adam Greenstein interviewed on LBC radio
In this paper, we studied a mouse model of mild hypertension, the polygenic BPH-2 mouse. The mice showed a reduction in brain blood flow along with clinical characteristics very similar to vascular dementia (apathy, executive dysfunction, mild memory loss). We found increased contractility of pial arteries due to a separation of the sarcoplasmic reticulum from the plasma membrane in vascular smooth muscle cells. This meant that although Ca2+ sparks were still present, they couldn’t activate the BK channel. The paper was featured in several national newspapers (see ‘In the news’) and on LBC national radio.

Taylor JL & Pritchard HAT et al. Functionally linked potassium channel activity in cerebral endothelial and smooth muscle cells is compromised in Alzheimer’s disease.
Proceedings of the National Academy of Sciences USA 2022;119(26):e2204581119. doi: 10.1073/pnas.2204581119
Weblink to Open access text
Listen to Adam Greenstein interviewed by Amol Rajan on BBC Radio 4
Here, we uncovered the mechanism to account for the reduction in brain blood flow in Alzheimer’s Disease. It had been known for around fifteen years that there was a reduction in blood flow to the brain in Alzheimer’s and moreover, people with a compromised brain blood flow appeared to have more severe disease and were more likely to have subsequent disease progression. We studied the arteries that traverse the surface of the brain (‘pial’ arteries) and found that they were tightly constricted due to damage to the function of a key vasodilating Potassium (K+)channel, the Calcium (Ca2+) activated large conductance K+channel–or ‘BK’.

When pial arteries are functioning normally, BK channels are activated by very small Ca2+ release events known as Ca2+ sparks. These Ca2+ spark events were discovered by Mark Nelson in 1995 and his paper is one of the most highly regarded publications in the field of small artery science. In the mice with Alzheimer’s, there was a reduction in Ca2+ sparks, accounting for the increased contractility of the arteries. We then went on to show that short term application of the protein Amyloidβ constricted healthy arteries by damaging BK channel function. These discoveries pointed to a potentially new approach to Alzheimer’s restoring BK channel function to improve brain blood flow in dementia with the aim of slowing the progress of the disease. The paper was picked up by online and print media across the world and Adam was interviewed by Amol Rajan on the Today programme.
Worton S.A. et al. Kynurenine relaxes arteries of normotensive women and those with pre-eclampsia. Circulation Research 2021;128:1679-1693 Weblink to open access text


In this paper, we studied small arteries taken from women undergoing elective and emergency Caesarian sections at St Mary’s Hospital. The arteries were isolated from a sample of tissue taken from the ‘omentum’; an apron-like structure which overhangs the bowel within the abdominal cavity. We would like to acknowledge their incredibly generous and selfless contribution to this important research. The study focuses on the molecule Kynurenine, which is a metabolite of trypophan and is known to be involved in blood pressure regulation. Stephanie (lead author of the paper) had previously suggested that Kynurenine might be a new treatment for hypertension but wished to see exactly how it worked. In the study, we found that for both normotensive and hypertensive women, kynurenine promoted relaxation of small arteries. We then used high speed Ca2+ imaging and electrophysiology to show that Kynurenine vasodilated arteries via activation of the vascular smooth muscle large-conductance Ca2+-activated K+ channels (BK), partly by influencing Ca2+ sparks.
Greenstein A.S. et al. Disruption of Pressure-induced Ca2+ spark vasoregulation of resistance arteries, rather than endothelial dysfunction underlies obesity related hypertension. Hypertension 2020;75(2):539-548. PMID: 31865779 Weblink to open access text
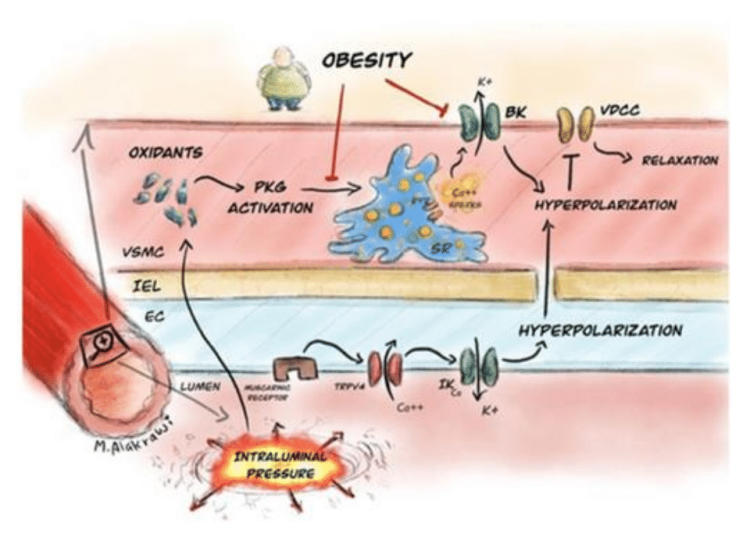

In this study, we discovered a new mechanism to account for why blood pressure goes up when people put weight on. We studied mice which had been put on a high fat diet and studied mesenteric arteries. We found the arteries to constrict much more strongly than those from mice fed on a normal diet. The cause of the enhanced constriction was damage to the function of a key vasodilating Potassium (K+) channel, the Ca2+ activated large conductance K+channel – or ‘BK’. When mesenteric arteries are functioning normally, BK channels are activated by very small Ca2+ release events known as Ca2+ sparks. In the high fat fed mice there was a lack of Ca2+ sparks, leading to the enhanced constriction. Searching for a cause for the loss of Ca2+ sparks, we examined a cellular pathway within vascular smooth muscle cells which we had discovered in 2016 – the activation of Protein Kinase G via stretch induced oxidant formation. We found that this pathway was disabled in the obese mice. The work identifies this novel pathway in vascular smooth muscle cells: intraluminal stretch due to pressure–generation of oxidants–PKG activation–release of Ca2+ sparks–vasodilation, as a new druggable target in the treatment of obesity related hypertension. In 2021, the paper was awarded ‘Best Basic Science’ paper by the journal ‘Hypertension’–the leading international journal in this field.
Koide M. et al. Differential restoration of functional hyperaemia by antihypertensive drug classes in hypertension-related cerebral small vessel disease. Journal of Clinical Investigation 2021;131(18):e149029. PMID: 34351870
In this 2021 paper, we showed that hypertension damages the process by which blood is supplied to the active areas of the brain – a mechanism called neurovascular coupling. We also then showed that a common anti-hypertensive drug called amlodipine was very effective at restoring this, as compared with other types of anti-hypertensive drugs. The paper was featured heavily in the international media with a full page article in The Times (see ‘In the news’).

The figure below, taken from the accompanying editorial by Professor Frank Faraci, illustrates that hypertension damages the function of the capillary endothelial inwardly rectifying potassium channel Kir2.1. This channel detects potassium which is released by active neurones and the subsequent change in membrane potential to the endothelial cell is transmitted against the flow of blood up to the feeder arteriole which then dilates. In hypertension, damage to Kir2.1 prevents this vasodilation in response to neural activity and this accounts for the development of brain damage and dementia in hypertension.

Pritchard H.A.T. et al. Nanoscale coupling of junctophilin-2 and ryanodine receptors regulates vascular smooth muscle cell contractility. Proceedings of the National Academy of Sciences USA 2019;116(43):21874-21881 PMID: 31591206 Weblink to full access text
